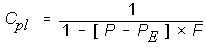We just installed a volumetric propane gas meter (temperature-compensated) installed on a boiler supply line. One pulse = 0.5 cubic meters. Our system runs @ 10PSI.
The boiler condition monitoring PLC that already measures steam flow rate will be used to determine how many pounds of steam is generated per unit of propane burnt.
Since we purchase propane by the liter (liquid), I would like to convert cubic meters @ 10 PSI to liters, but can't find a conversion table or formula. I did find one that says "Specific volume (1.013 bar and 21 °C (70 °F)) : 0.543 m3/kg". It's easy to convert kg to L, but how do I allow for the pressure differential? This formula is for 14.7PSI (1.013 bar), but I need 10 PSI.
Can anyone help me out with this?
The boiler condition monitoring PLC that already measures steam flow rate will be used to determine how many pounds of steam is generated per unit of propane burnt.
Since we purchase propane by the liter (liquid), I would like to convert cubic meters @ 10 PSI to liters, but can't find a conversion table or formula. I did find one that says "Specific volume (1.013 bar and 21 °C (70 °F)) : 0.543 m3/kg". It's easy to convert kg to L, but how do I allow for the pressure differential? This formula is for 14.7PSI (1.013 bar), but I need 10 PSI.
Can anyone help me out with this?
Last edited: