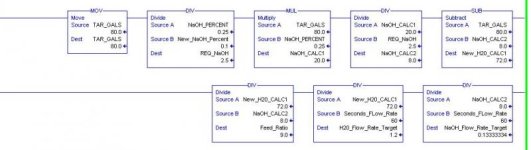
problem
I have a solution of 25% sodium and 75% H20
During transfer I want to reduce the 25% to a ratio of
10% sodium and 90% water
If the total transfer amount is 80 gallons the
the original targets would be 20 gallons of sodium and
60 gallons of water based on the 25% solution.
So here is were I am lost
I would need to increase the water amount by ?? to maintain the
80 gallons at 10%.
For some reason I just am not getting it
I have a solution of 25% sodium and 75% H20
During transfer I want to reduce the 25% to a ratio of
10% sodium and 90% water
If the total transfer amount is 80 gallons the
the original targets would be 20 gallons of sodium and
60 gallons of water based on the 25% solution.
So here is were I am lost
I would need to increase the water amount by ?? to maintain the
80 gallons at 10%.
For some reason I just am not getting it